So What’s a Cavity, Anyways?
Posted: August 1, 2011
Last Modified: September 21, 2020
Everybody knows what a cavity is, right? When you get right down to it, it turns out that very few people actually know how a cavity is formed. This is important, because it goes to the heart of managing (and preventing) cavities. So let’s explore this most basic of dental problems…
A cavity is a hole in a tooth caused by repeated acid dissolution of that area of the tooth. (If you want an official definition, here it is.) Wait – what? What’s the difference between caries and a cavity?
Let’s start by understanding what a tooth is like when healthy. The bulk of a tooth is made up of dentin, with a layer of enamel covering the top of the tooth (the crown) and a thin layer of cementum covering the bottom (root). The very centre of a normal tooth is composed of a mushy, pulp-like mass containing blood vessels, fibrous tissue, and most familiarly, nerve tissue. The very descriptive term pulp is given to this portion of the tooth.
See here for a labeled photograph of a normal tooth, albeit caked with tartar (they really could have cleaned the tooth off before taking the photo).
See here for a cutaway diagram of a normal tooth.
Under normal circumstances, only the crown of the tooth (the part covered by enamel) is exposed to the oral cavity. Enamel is the hardest material in the body, and needs to be that way in order to tolerate the forces of chewing. Composed almost entirely of mineral (modified hydroxyapatite crystals), enamel has the ability to rebuild itself after being dissolved by acids. This is not unlike other crystals that you may have grown as a childhood science experiment – see crystals growing here.
(Please see one of our previous blog posts about acids in the mouth).
While dietary acids dissolve away enamel crystals, minerals in the saliva, including Ca2+and PO43- (calcium and phosphate) are able to rebuild the lost enamel so that the tooth is not forever getting smaller. However, this can only happen when the pH of the dissolved area is above a certain level (higher than pH 5.5).
So, the tooth undergoes repeated cycles of dissolution (demineralization) and rebuilding (remineralization) as the pH drops from dietary acids and rises again as the natural buffering of the saliva brings the pH back up.
But what happens if the pH is kept below 5.5? The enamel crystals continue to dissolve away, and if this continues (this is the caries we were talking about), eventually the entire thickness of the enamel is compromised and it actually caves in. Now, instead of mere caries, it has progressed to a full-fledged cavity. We have now reached the point of no return, where the enamel is no longer able to rebuild, and the cavity must be addressed with a filling.
At later stages, this is what a cavity looks like.
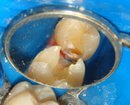
So there you have it – a sustained period of acid dissolution that causes so much demineralization of the tooth that it caves in. Isn’t that what you were going to say?
Keeping good hygiene and minimizing the snacking or eating times will maximize the amount of remineralization time, and allow the teeth to repair themselves!
Please contact us for a personalized assessment of your teeth, including your caries risk! Thanks for reading.


